by Art Writ, November 17th, 2011
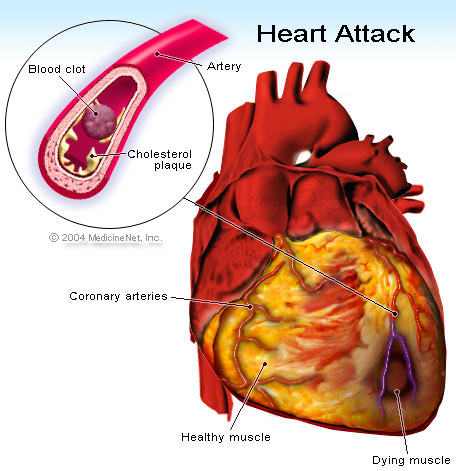
Heart attack, or medically termed as Myocardial infarction (MI) or acute myocardial infarction (AMI), is a result of the disruption of blood supply in the heart cells leading to absence of oxygen that supplies the heart. As a result, the heart tissues become damaged and scar. The common cause of heart attack is the blockage or occlusion of the coronary arteries that carries blood supply to the heart. Often times, this condition is brought about by a vulnerable atherosclerotic plaque, which is an unstable collection of lipids (cholesterol and fatty acids) and white blood cells (especially macrophages) in the wall of an artery.
University of North Carolina (UNC) at Chapel Hill School of Medicine conducted a new study signifying that the scar formation in the heart tissues is necessary in the healing process of healing the damaged part of the heart. They also suggest that interrupting this process can lead to a weaker heart. The findings of a new study were presented last 15 November online issue of The EMBO Journal.
Previously, it is believed that the scarring of the heart tissues following a heart attack hardens the walls of the heart. Due to this, the hardening will contribute to the less flexibility and pumping force of the heart. In return, there will be less blood that the heart can be pumped out of the heart gong to the systemic circulation. Until this study was conducted, findings changed the old one. The senior author Dr Arjun Deb, an assistant professor of medicine and cell and molecular physiology at the UNC School of Medicine, and colleagues, found that blocking cells in the outer layer of the mammalian heart from generating scar tissue can quickly lead to heart failure as demonstrated in lab mice. They concluded that, it was not that scarring should be allowed to run its course completely, but more a case of timing intervention to achieve the right balance between the tissue regeneration that scarring triggers, and reducing the damage of scarring. Deb, who is also a member of UNC’s McAllister Heart Institute and the Lineberger Comprehensive Cancer Center, expresses to the media:”We now know that scarring is a good thing, because it prevents a precipitous decline in heart function immediately after heart injury.” “The question is not whether, but when it makes the most sense to manipulate the cells of the heart to decrease scarring and enhance regeneration,” said Deb.
Furthermore, the researchers decided to investigate what happens to the epicardium, the outer layer of the heart, of the mammalian heart after injury, like a heart attack. They found out that the epicardium play a role in the formation of fibroblost, the cells that underlie the scar tissue. Unlike in the lower organisms like the zebrafish, the epicardium directly contributes to the formation and regeneration of the heart muscle cells. The protein called Wnt1 was also discovered to drive the stem cells in the epicardium to become fibroblast. They supposed, if they interrupted Wnt1 signalling, the activity would shift from boosting fibroblasts to making blood vessel cells, thus reducing scarring and helping regeneration and heart function.
But when they applied this in genetically engineered mice just after cardiac injury, they developed heart failure within 2 weeks. Deb said it was clear that some “evolutionary parallels” exist between zebrafish and mice, but there must be an advantage, a selection pressure, for mammals, unlike lower organisms, to have a response to heart injury that leads to scarring, because interrupting this quickly leads to heart failure. He added:”In organisms where there is a high pressure of blood flow, these cells may need to turn into scar tissue to maintain the tensile strength of the heart wall and prevent catastrophic rupture.”
Presently, the team is experimenting with intervening in the healing process at various different times after injury: manipulating the stem cells in the epicardium at different points in time to see if they can persuade them not to become fibroblasts and become heart-regenerating myocytes instead hoping to generate a better result that can be useful in patients recovering from heart attack.